All cats are gray in the dark
An astronomical view on the physiology of vision

| rhodopsin activation | dark adaptation | rods vs. cones |
|
All cats are gray in the dark An astronomical view on the physiology of vision |
 |
If you search the astronomy related internet forums about vision and in particular dark and light adaptation and its relation to deep sky observing, you will find the wildest theories. What precisely is rhodopsin and what does it do? How does vision at night differ from that at daylight? What is dark adaptation and why does it take so long? And how is this all connected to red light?
During my time as a researcher at the University of Freiburg, I worked for many years as a physicist with spectroscopic methods on the activation and deactivation processes of rhodopsin (here is list of publications of that time). For our annual Deep Sky Meeting 2012, I put together in talk those topics on vision that I felt the most important for us visual observers. In the following three part essay, I would like to present the essentials of that talk.
part 1
Rhodopsin activation
| Anatomy of the
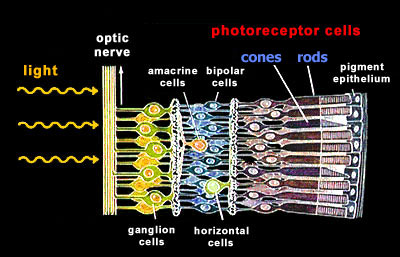
retina Our retina has two general types of photoreceptor cells, the rods and the cones. Unexpectedly and mostly for evolutionary reasons, these photoreceptor cells are localized at the back of our retina. Light needs to traverse several layers of nerve cells before it reaches the light-sensitive photoreceptor cells.
|
 |
|
Three types of cones, one type of
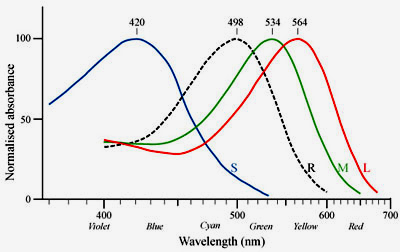
rods The photoreceptor cells can be further subdivided according to their spectral sensitivities: There is only one type of rod cells (R) that is responsible for dim light (scotopic) vision and that has its peak sensitivity in the green range at around 500nm. There are three types of cones, S (blue), M (green), and L (red), that are responsible for color vision during daylight (photopic vision). |
| Design
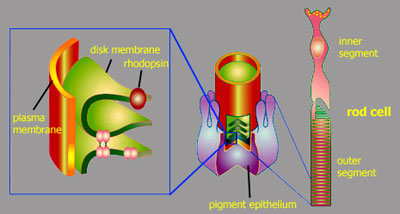
of a rod cell Let's take a closer look at a rod cell. The rod photoreceptor cells consist of an inner segment with the nucleus and an outer segment. These outer segments contain stacks of membrane vesicles termed disk membranes, that are densely packed with the actual photoreceptor in the cell, the visual pigment rhodopsin.
|
 |
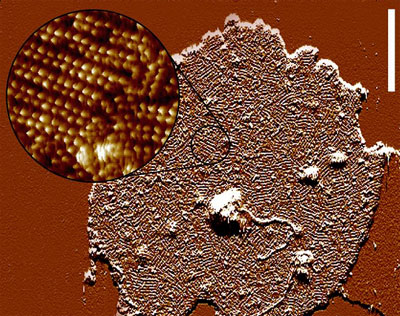
This The atomic force microscopic image to the right gives a good impression of the extremely high receptor density in the disk membrane (each little bump corresponds to a single rhodopsin). The quasi-crystalline structure that is indicated in this image (Fotiadis et al. (2003) Nature 421:127) is very likely an artifact of sample preparation and does not correspond to the situation in the photoreceptor cell in vivo, where rhodopsin is known to have a relatively high coefficient of rotational diffusion. The high receptor density and the dense stacking of these disk membranes allows for a high probability to catch as many of the incoming photons as possible. |
 |
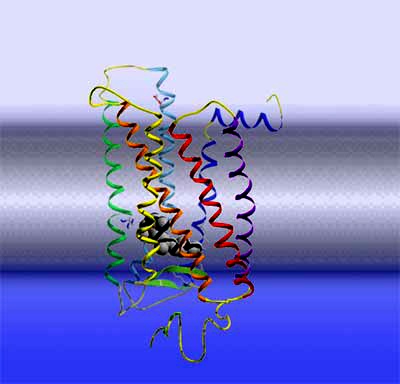 |
Rhodopsin Let's take another step deeper into the cell and look at a single rhodopsin molecule. To the right is a ribbon model of the actual light receptor, rhodopsin. Rhodopsin is a membrane protein, consisting of 7 membrane-spanning helices. Rhodopsin further belongs to a large and important class of membrane receptors termed "G protein-coupled receptors". This receptor family is the target of about 50% of all pharmaceuticals and hence the exploration of their structure and function is one of the big topics in life sciences. Members of this family are receptors for adrenaline, dopamine, other neurotransmitters, a large number of hormones, odorants, etc. As soon as one of these receptors bind its cognate ligand outside the cell, the receptor becomes activated and puts forward the signal in an intracellular signaling cascade. By the way, Robert Lefkowitz and Brian Kobilka were awarded the 2012 Nobel Prize in Chemistry for their groundbreaking work on this type of receptors.
|
|
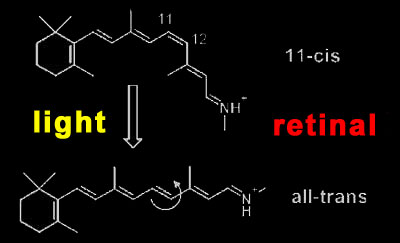
Rhodopsin activation Rhodopsin is a quite special member of this receptor family, as it has its light-sensitive ligand, the vitamin A derivative retinal, permanently bound. It is buried in the retinal binding pocket of rhodopsin as a kinked 11-cis isomer that is covalently bound to the protein via a protonated Schiff base. Due to its special electronic structure, it lends the photoreceptor its 500 nm absorption maximum (slightly longer for human rhodopsin). When retinal absorbs an incoming photon, it converts in one of the fastest (200fs) and most efficient (67% quantum yield) photochemical reactions at all to the straight all-trans form.
|
 |
 Ye et al. and Vogel (2010) Nature 464:1386
|
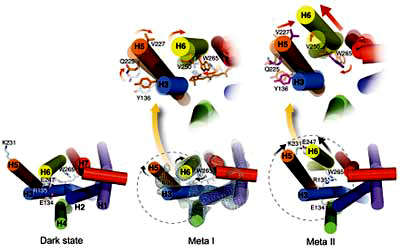
The subsequent steps
are complicated. The straight all-trans retinal no longer fits in its
binding pocket and forces the receptor protein to adopt a different
conformation. This does not happen in one step, but via a number of steps
and several photo intermediates. Each intermediate involves changes from its
predecessor and these changes occur hierarchically, covering ever larger
parts of the receptor protein. On the first levels, single amino acid side
chains bend and rotate and move, while on the latest levels, entire
α-helices
do rotate and move (see the figure to the left taken of one of my papers).
These changes also involve the breaking and closing of electrostatic
interactions between charged and polar groups or their neutralization via
proton uptake and transfer. The intermediates involved with the activation
of rhodopsin are termed Batho, BSI, Lumi, Meta I, and finally Meta II.
|
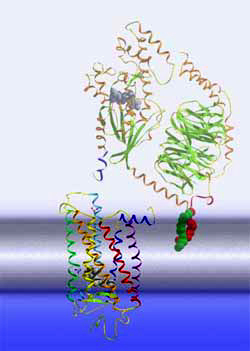
| G
protein activation cascade The Meta II state corresponds to the active receptor conformation, R*. In Meta II, the light-induced changes in the retinal binding pocket have propagated over the entire receptor protein and have re-arranged the cytoplasmic side of the receptor, such that it can now bind another protein, a G protein. This binding induces a nucleotide exchange in the G protein and the activation of the G protein itself. Once activated, the G protein leaves its docking place at the receptor, giving way for the activation of further G proteins and the first amplification of the light signal. The activated G proteins bind then to further effectors downstream the signaling cascade, involving further amplification steps.
|
 |
| One of the paradigms
of signal transduction is a high signal-to-noise ratio. This means as much
signal as possible with as little noise as possible.
A high signal is achieved by:
Low noise is achieved by:
|
read on Dark adaptation: What happens in our eye when it's getting dark?
![]()
| rhodopsin activation | dark adaptation | rods vs. cones |